Mataupu
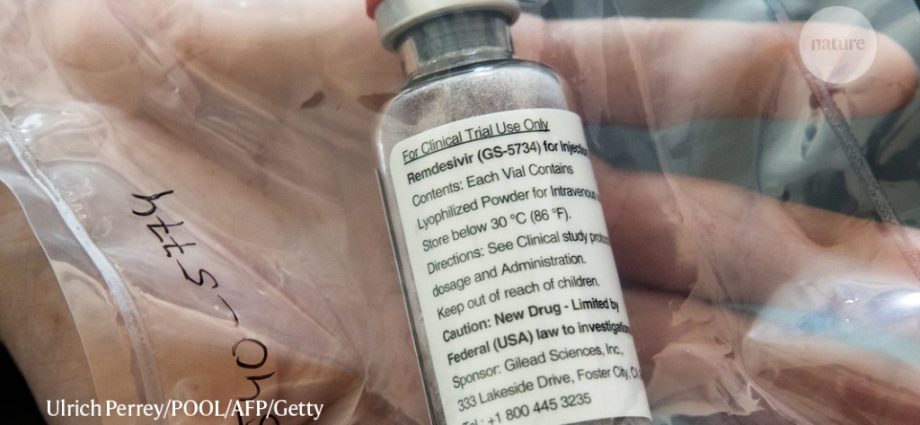
Remdesivir is an antiviral drug given to patients with SARS-CoV-2 infections. So far, it is the only agent used to treat COVID-19, officially approved by US and European drug approval agencies. According to information from the Ministry of Health, over 100 were ordered in April. pieces of remdesivir, several times more than in the previous months. However, according to doctor Bartosz Fiałek, it is difficult to estimate whether it is a sufficient amount.
- Remdesivir is an antiviral medicine originally developed to fight the Ebola virus
- Currently, it is administered in hospitals to patients infected with the coronavirus, whose saturation levels are falling
- The demand for remdesivir is constantly growing, which is why the Ministry of Health has recently increased the order significantly
- The drug is not used in every hospital, and moreover – we do not know how many people require remdesevir therapy – emphasizes doctor Bartosz Fiałek
- Mo nisi tala coronavirus, siaki le itulau autu o TvoiLokony
Remdesivir allows to shorten the hospitalization time of COVID-19 patients
Remdesivir is still the only drug currently used to treat COVID-19 patients. Despite the fact that from time to time information about effective therapy with other agents comes in, they still haven’t got the green light when it comes to mass and official treatment.
Remdesivir is the only drug approved by the US Food and Drug Administration (US Food and Drug Administration) and then the EMA (European Medicines Agency) for use in people from 12 years of age in patients with COVID-19 pneumonia who require oxygen, says Bartosz Fiałek, a doctor.
Many other drugs are under investigation, such as monoclonal antibodies, cocktails made of these antibodies, such as REGN-COV2, which was given to former US President Donald Trump. There are glucocorticosteroids, such as dexamethasone, which also have a positive effect on the course of COVID-19, i.e. reduce the risk of death due to the severe course of the disease. There are also drugs that act against blood clotting, such as low molecular weight heparins, or anticoagulants. Apart from remdesivir, which is approved for use in the treatment of COVID-19, the other drugs mentioned are conditionally approved, i.e. for emergency use (EUA), adds Fiałek.
- O se vailaʻau mo le COVID-19 e maualuga le faʻamoemoe o fomaʻi. O le isi su'esu'ega manuia
– Remdesivir was developed to fight Ebola and has also been shown to be an effective antiviral drug in reducing the risk of dying from COVID-19 and shortening hospitalization time from an average of 15 to an average of 11 daysi. So you can see that the drug affects the course of the disease. Remdesivir combined with glucocorticosteroids or monoclonal antibodies may allow for the development of a possible therapeutic model that will help many patients. At the present stage, however, we do not have a causal drug available to treat COVID-19, as, for example, in the case of streptococcal angina, it is an antibiotic of the penicillin group. Hence, a large number of deaths – but less than placebo – in people who received remdesivir, explains the specialist in rheumatology.
We asked the Ministry of Health what the stocks of remdesivir are currently looking like.
«In the last 4 months, 148 jobs have been delivered to Poland. of the drug, including 52 thousand in March alone. In April, we are to receive 102 thousand. We have definitely increased orders, but unfortunately Gilead is not able to increase production capacity to meet the needs of all comers, and this is the only manufacturer of the drug »- we read in the information sent by the Ministry of Health Communications.
- “In 10 days we may have a thousand deaths from COVID-19”
As you can see, the order for the next month is much larger than in the previous one, but is this enough of this drug? – Hard to say. The resources that MZ talks about are impossible to comment on, because I would have to know the statistics of hospital needs. The drug is not used in every hospital, and moreover – we do not know how many people require treatment with remdesevir.It is rather reading tea leaves. The situation is dynamic. 100 thousand. ordered pieces for 5 thousand. infections, and differently with 35 thousand. It is impossible to assess how many people end up in hospitals that have remdesivir in their treatment resources. Covid hospitals probably do, but there are also departments in poviat hospitals that accept people infected with SARS-CoV-2, where the drug may not be available, says doctor Bartosz Fiałek.
The Ministry of Health also does not have statistics. There are no specific guidelines for use, we have only learned that “the decision is made by the doctor treating the patient in the hospital”.
- Coronavirus i Polani - fa'amaumauga mo voivodeships [FA'AMATALAGA NEI]
– These 100 thousand it may not be sufficient if it were to be administered wherever COVID-19 patients are treated. First of all, look at the dosage form of the drug – 1 vial contains 100 mg of the drug, and the patient is given 200 mg on the first day and then 100 mg for up to 10 days (possibly shorter, it all depends on the patient’s clinical condition) – continues Fiałek.
– However, a significant increase in the size of the purchase of remdesevir may indicate that the Ministry of Health is aware of the scale of the epidemic tragedy – concludes the doctor.
Faitau foi:
- E toafia tagata i Polani na maliliu ina ua maeʻa le tui COVID-19? Fa'amaumauga a le Malo
- Ua fa'ateleina ma'i talavou i falema'i ona o le COVID-19
- E ta'u atu e foma'i pe fa'apefea ona e iloa pe ua tu'ua e le COVID-19 ni fa'ailoga i lou tino
- Ituaiga o tui COVID-19. E fa'afefea ona 'ese'ese le veki mai le tui mRNA? [MATOU FAAMANATU]
O mea o loʻo i totonu o le upega tafaʻilagi medTvoiLokony ua faʻamoemoe e faʻaleleia, ae le o le suia, le fesoʻotaʻiga i le va o le Tagata Faʻaoga Upega Tafaʻilagi ma a latou fomaʻi. O le upega tafa'ilagi ua fa'amoemoe mo na'o fa'amatalaga fa'aa'oa'oga. Aʻo leʻi mulimuli i le poto faʻapitoa, aemaise fautuaga faʻafomaʻi, o loʻo i luga o la matou Upega Tafaʻilagi, e tatau ona e faʻafesoʻotaʻi se fomaʻi. E leai se a'afiaga a le Pule e mafua mai i le fa'aogaina o fa'amatalaga o lo'o i luga ole Upega Tafa'ilagi. E te mana'omia se fa'atalanoaga fa'afoma'i po'o se fa'amatalaga fa'akomepiuta? Alu i le halodoctor.pl, e te maua ai le fesoasoani i luga ole laiga – vave, saogalemu ma e aunoa ma le tuua o lou fale.Ole taimi nei e mafai ona e fa'aogaina e-consultation e aunoa ma se totogi i lalo ole National Health Fund.